Advertisement
The leading life science news channel in the Nordic region.

Clinical Trials - February 8, 2023
Evaxion Biotech and Pantherna Therapeutics have announced preclinical proof of concept for the combination of the two companies’ key technologies. The preclinical data demonstrate that tumor neoantigens identified by Evaxion’s AI platform, PIONEER, drive a strong immune response and lead to complete inhibition of tumor growth in a preclinical model when delivered using Pantherna’s lipid […]

Pharma Business - February 8, 2023
Orexo has announced the submission of a New Drug Application to the US Food and Drug Administration for its lead pharmaceutical pipeline asset, OX124, a nasal rescue medication for opioid overdose. OX124, is based on Orexo’s drug delivery platform amorphOX and contains a high-dose of naloxone. “With the filing of OX124 we are now one […]

Pharma Business - February 7, 2023
Forxiga has been approved in the European Union to extend the indication for heart failure (HF) with reduced ejection fraction (HFrEF) to cover patients across the full spectrum of left ventricular ejection fraction (LVEF), including HF with mildly reduced and preserved ejection fraction (HFmrEF, HFpEF). “This broader indication for Forxiga for the treatment of symptomatic […]

Biotech Business - February 7, 2023
The financing was led by Finland’s Lifeline Ventures, an early-stage venture capital firm founded by serial entrepreneurs. This follows on from the first close of 10 million EUR in June 2022. This final close of 12 million EUR includes 5.9 million EUR equity from the European Innovation Council (EIC) Fund as well as a 2.1 […]

Pharma Business - February 1, 2023
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended darolutamide, an oral androgen receptor inhibitor (ARi), plus ADT in combination with docetaxel for marketing authorization in the European Union (EU) for the treatment of patients with metastatic hormone-sensitive prostate cancer (mHSPC). Darolutamide is already approved under the […]
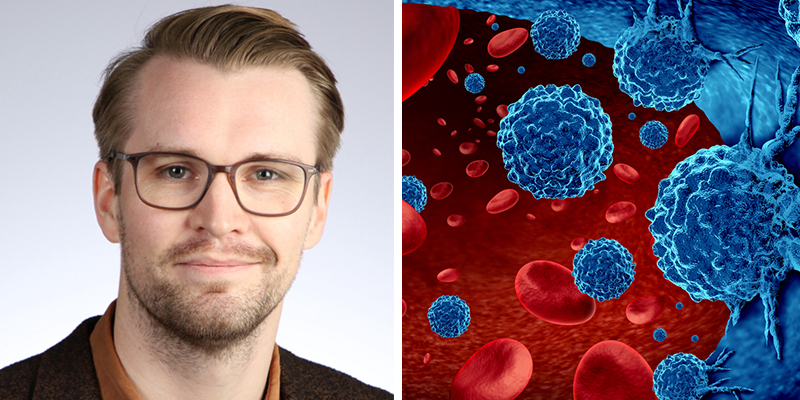
Science - February 1, 2023
Findings from the major study iStopMM has been used to redefine monoclonal gammopathy of undetermined significance (MGUS), a precursor to multiple myeloma. We spoke with Þórir Einarsson Long, one of the authors of Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study, published in […]

Pharma Business - February 1, 2023
Lundbeck and Otsuka Pharmaceutical have announced that the FDA has determined that the supplementary New Drug Application (sNDA) for brexpiprazole for the use in the treatment of agitation associated with Alzheimer’s dementia (AAD) is sufficiently complete to permit a substantive review. The FDA has assigned the application priority review and a Prescription Drug User Fee Act […]

Business - February 1, 2023
The last patient has now been included in the ongoing clinical study with ACD440, the lead non-opioid drug candidate in the painless platform, which is being developed against peripheral neuropathic pain. This double-blind, placebo-controlled, randomized cross-over study, which is carried out in collaboration with LINK Medical Research in Uppsala, is aimed at evaluating the efficacy, […]

Business - February 1, 2023
Nordic Biomarker was recently named ‘Exporter of the Year 2022’ but the Umeå-based company says its journey has only just begun. The company has announced its vision to become a global force in the life science industry – with an ambitious five-year goal to reach almost a 1 billion SEK turnover and potentially employ over […]

Biotech Business - January 30, 2023
Just before Christmas, the Norwegian company announced that it has received support from Oslo County of 1.950 million NOK. The funding will support optimization of the synthesis of our two lead compounds, APC148 and APC247. The program will define the optimal characteristics of the substances that will be used for microbiology testing as well as […]
This site uses cookies