Advertisement
The leading life science news channel in the Nordic region.

Agreement - May 24, 2023
The two companies have announced a research and development collaboration to discover and develop gene editing therapies against a select set of therapeutic targets. With a focus on advancing base editing capabilities, Novo Nordisk will leverage Life Edit’s suite of gene editing technologies to precisely edit the genome with the aim of developing life-changing therapies […]

Clinical Trials - May 24, 2023
AlzeCure Pharma has received positive data from the clinical study with the non-opioid drug candidate ACD440, which is developed against peripheral neuropathic pain. The phase II data show that ACD440 was able to demonstrate positive proof-of-mechanism (PoM) results in patients with chronic peripheral neuropathic pain, i.e. the drug candidate has an effect on the intended […]

Financing - May 24, 2023
The money has been raised for additional development activities of their product pHyph for antibiotic free treatment of vaginal infections. The funds will be used for an ongoing proof-of-concept study for the treatment of Vulvo Vaginal Candidiasis (VVC) with pHyph, partially financed by a recent grant from Swelife and Medtech4Health, investigating how the vaginal microbiome […]

Biotech Business - May 23, 2023
BioLamina announces the release of the first Biolaminin 521 LN batches issued from its new manufacturing facility in Sundbyberg, Sweden. The products, which are now ready for commercialization, have been produced in the latest expansion of BioLamina premises. This facility is dedicated to production and research within process development, matrix biology and cellular assays. The […]
Clinical Trials - May 23, 2023
Spago Nanomedical has announced that the final step in the preparations for the clinical phase I/IIa trial in cancer patients, Tumorad-01, with its leading candidate drug 177Lu-SN201 has begun in Australia. The application to start the study has been sent to the ethics committee and patient enrollment is expected to start in the summer 2023. […]

Clinical Trials - May 23, 2023
Valo Therapeutics has announced that the first patient has been treated in its trial of PeptiCRAd-1 (Peptide-coated Conditionally Replicating Adenovirus) in three tumor types. PeptiCRAd-1 combines two clinically proven cancer immunotherapy approaches: oncolytic adenoviruses and tumor-specific peptides for the generation of strong systemic cytotoxic T-cell responses against multiple tumor antigens. This is achieved by coating […]

Pharma Business - May 22, 2023
Genmab has announced that the U.S. Food and Drug Administration (FDA) has approved EPKINLY (epcoritamab-bysp) as the first and only T-cell engaging bispecific antibody for the treatment of adult patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), including DLBCL arising from indolent lymphoma, and high-grade B‑cell lymphoma, after […]

Intellectual Property - May 22, 2023
AlzeCure Pharma has announced that the European Patent Office (EPO) has granted a patent covering the company’s candidate drug ACD856, which is developed against Alzheimer’s disease and other disorders with cognitive impairment. “This is another key step for ACD856, given that we have previously also been granted a US patent for this substance. This further […]

Agreement - May 17, 2023
IRLAB Therapeutics has announced the signing of an agreement, with the McQuade Center for Strategic Research and Development (MSRD), a member of the global Otsuka family of pharmaceutical companies. Under the agreement MSRD will evaluate the neuropsychiatric compounds IRL757 and IRL942 under exclusivity. MSRD, which identifies and supports early-stage opportunities that can change the landscape […]
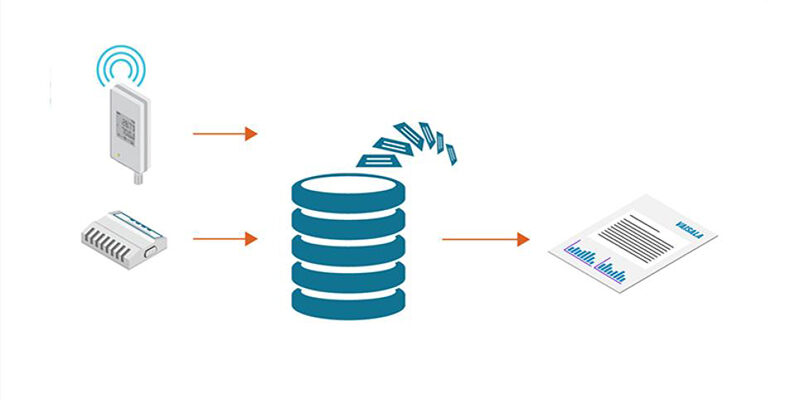
Sponsored content - May 16, 2023
A document exists within a system, and in the case of GxP environments, that system is tightly controlled. In the case of Vaisala’s viewLinc monitoring system, the GMP data is always in viewLinc’s database. This is a question that came up during our webinar on Data Integrity. In this blog, we answer at length. This […]
This site uses cookies